Amenorrhea (the absence of menstruation) can be primary or secondary.
Primary amenorrhea is failure of menses to occur by age 15 years in patients with normal growth and secondary sexual characteristics. However, absence of any breast development by age 13 should prompt evaluation for primary amenorrhea.
Secondary amenorrhea is the absence of menses for ≥ 6 months or for the length of 3 cycles after the establishment of regular menstrual cycles (1, 2). However, patients with previously regular cycles are evaluated for secondary amenorrhea if menses have been absent for ≥ 3 months, and patients with previously irregular cycles are evaluated for secondary amenorrhea if menses have been absent for ≥ 6 months.
Normally, the hypothalamus generates pulses of gonadotropin-releasing hormone (GnRH). GnRH stimulates the pituitary to produce gonadotropins (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]—see figure The idealized cyclic changes in pituitary gonadotropins, estradiol, progesterone, and uterine endometrium during the normal menstrual cycle), which are released into the bloodstream. Gonadotropins stimulate the ovaries to produce estrogen (mainly estradiol), androgens (mainly testosterone), and progesterone. These hormones do the following:
If pregnancy does not occur, estrogen and progesterone production decreases, and the endometrium breaks down and is sloughed during menses. Menstruation occurs 14 days after ovulation in typical cycles.
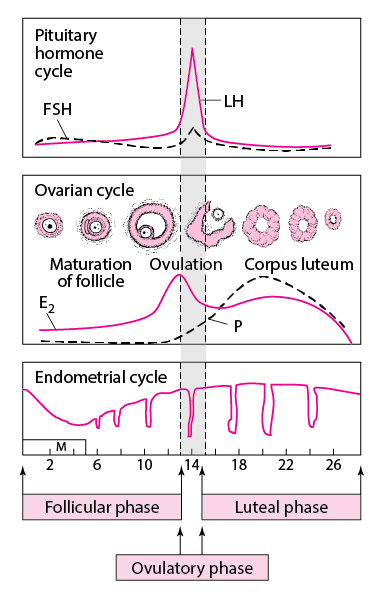 |
When part of this system malfunctions, ovulatory dysfunction occurs; the cycle of gonadotropin-stimulated estrogen production and cyclic endometrial changes is disrupted, resulting in anovulatory amenorrhea, and menstrual flow may not occur. Most amenorrhea, particularly secondary amenorrhea, is anovulatory.
However, amenorrhea can occur when ovulation is normal, as occurs when genital anatomic abnormalities (eg, congenital anomalies causing outflow obstruction, intrauterine adhesions [Asherman syndrome]) prevent normal menstrual flow despite normal hormonal stimulation.
Amenorrhea can be classified based on a number of different criteria, such as
Anatomic causes can generally be identified by physical examination.
For general clinical evaluation, it is useful to classify amenorrhea as follows:
Each type of amenorrhea has many causes, but overall, the most common causes of amenorrhea include
Contraceptives can cause the endometrium to thin, sometimes resulting in amenorrhea; menses usually begin again about 3 months after stopping oral contraceptives.
Antidepressants and antipsychotics can elevate prolactin, which stimulates the breasts to produce milk and can cause amenorrhea.
Some disorders can cause ovulatory or anovulatory amenorrhea. Congenital anatomic abnormalities cause only primary amenorrhea. All disorders that cause secondary amenorrhea can cause primary amenorrhea.
The most common causes of anovulatory amenorrhea involve a disruption of the hypothalamic-pituitary-ovarian axis. Thus, causes include
Women with amenorrhea due to hypothalamic dysfunction have lower levels of serum leptin (an anorectic hormone produced by fat cells); lower levels may contribute to decreased gonadotropin production.
Anovulatory amenorrhea is usually secondary but may be primary if ovulation never begins—eg, because of a genetic disorder. If ovulation never begins, puberty and development of secondary sexual characteristics are abnormal. Genetic disorders that confer a Y chromosome increase the risk of ovarian germ cell cancer.
 |
TABLESome Causes of Anovulatory Amenorrhea

The most common causes of ovulatory amenorrhea include
TABLESome Causes of Ovulatory Amenorrhea

Obstructive abnormalities are usually accompanied by normal hormonal function. Such obstruction may result in
Because ovarian function is normal, external genital organs and other secondary sexual characteristics develop normally. Some congenital disorders (eg, those accompanied by vaginal aplasia or a vaginal septum) also cause urinary tract and skeletal abnormalities.
Some acquired anatomic abnormalities, such as endometrial scarring after instrumentation for postpartum hemorrhage or infection (Asherman syndrome), cause secondary ovulatory amenorrhea.
Girls are evaluated for primary amenorrhea if
Girls and women of reproductive age should be evaluated for secondary amenorrhea if they have previously been menstruating and have
Evaluation of secondary amenorrhea should always include a pregnancy test.
History of present illness includes the following:
Review of systems should cover symptoms suggesting possible causes, including the following:
Patients with primary amenorrhea are asked about symptoms of puberty (eg, breast development, growth spurt, presence of axillary and pubic hair) to help determine whether ovulation has occurred.
Past medical history should note risk factors for the following:
Drug history should include specific questions about use of drugs, such as the following:
Family history should include height of family members and any cases of delayed puberty or genetic disorders in family members, including Fragile X syndrome.
Clinicians should note vital signs and body composition and build, including height and weight, and should calculate body mass index (BMI). Secondary sexual characteristics are evaluated; breast and pubic hair development are staged using the Tanner method. If axillary and pubic hair is present, adrenarche has occurred.
With the patient seated, clinicians should check for breast secretion by applying pressure to all sections of the breast, beginning at the base and moving toward the nipple. Galactorrhea (breast milk secretion not temporally associated with childbirth) may be observed; it can be distinguished from other types of nipple discharge by finding fat globules in the fluid using a low-power microscope.
Pelvic examination is done to detect anatomic genital abnormalities (eg, imperforate hymen, vaginal septum, vaginal, cervical, or uterine aplasia). A bulging hymen may be caused by hematocolpos, which suggests genital outflow obstruction. Pelvic examination findings also help determine whether estrogen has been deficient. In postpubertal females, thin, pale vaginal mucosa without rugae and pH > 6.0 indicate estrogen deficiency. The presence of cervical mucus with spinnbarkeit (a stringy, stretchy quality) usually indicates adequate estrogen. Clinicians should check for enlargement of the uterus, ovaries, and clitoris.
General examination focuses on evidence of virilization, including hirsutism, temporal balding, acne, voice deepening, increased muscle mass, clitoromegaly (clitoral enlargement), and defeminization (a decrease in previously normal secondary sexual characteristics, such as decreased breast size and vaginal atrophy). Virilization results from increased androgen production by the adrenal glands and ovaries. Hypertrichosis (excessive growth of hair on the extremities, head, and back), which is common in some families, is differentiated from true hirsutism, which is characterized by excess hair on the upper lip and chin and between the breasts.
Skin discoloration (eg, yellow due to jaundice or carotenemia, black patches due to acanthosis nigricans) should be noted.
Clinicians should check for hypothermia, bradycardia, hypotension, and reduced subcutaneous fat, which suggest anorexia nervosa, and for dental erosion, palatal lesions, reduced gag reflex, subconjunctival hemorrhage, and subtle hand changes with calluses on the dorsum of the hand (due to frequent vomiting), which suggest bulimia.
The following findings are of particular concern:
| If amenorrhea occurs in girls with secondary sexual characteristics or in women of reproductive age, do a pregnancy test regardless of sexual and menstrual history. |
In primary amenorrhea, the presence of normal secondary sexual characteristics usually reflects normal hormonal function; amenorrhea is usually ovulatory and typically due to a congenital anatomic genital tract obstruction. Primary amenorrhea accompanied by abnormal secondary sexual characteristics is usually anovulatory (eg, due to a genetic disorder).
In secondary amenorrhea, clinical findings sometimes suggest a mechanism (see table Findings Suggesting Possible Causes of Amenorrhea):
TABLEFindings Suggesting Possible Causes of Amenorrhea

History and physical examination help direct testing.
Pregnancy should not be excluded based on history; a pregnancy test is required. High-sensitivity urine tests should be done, and occasionally, a blood test may be required. Results are usually accurate several days before a missed menstrual period and often as early as several days after conception. Some over-the-counter (OTC) tests are less sensitive and accurate.
If girls have secondary sexual characteristics, a pregnancy test should be done to exclude pregnancy and gestational trophoblastic disease as a cause of amenorrhea. Women of reproductive age should have a pregnancy test after missing one menses.
The approach to primary amenorrhea (see algorithm Evaluation of primary amenorrhea) differs from that to secondary amenorrhea (see algorithm Evaluation of secondary amenorrhea), although no specific general approaches or algorithms are universally accepted.
If patients have primary amenorrhea and normal secondary sexual characteristics, testing should begin with pelvic ultrasonography to check for congenital anatomic genital tract obstruction. MRI may be needed if abnormalities are identified.
![Evaluation of primary amenorrhea [a]](https://www.msdmanuals.com/-/media/manual/professional/images/g/y/n/gyn_primary_amenorrhea_algorithm_no_fnts_change.gif?thn=0&sc_lang=en) |
| [a] Normal values areDHEAS: 250–300 ng/dL (0.7–0.8 mcmol/L)FSH: 5‒20 IU/LLH: 5‒40 IU/LKaryotype (female): 46,XXProlactin: 50 ng/mL (see below)Testosterone: 20–80 ng/dL (0.7–2.8 nmol/L)Although these values are representative, normal ranges may vary between laboratories.Prolactin 50–100 ng/mL is considered mildly elevated and is usually due to use of a drug. Prolactin > 100 ng/mL is considered elevated and is more likely to be due to a tumor. |
| [b] Some clinicians measure LH levels when they measure FSH levels or when FSH levels are equivocal. |
| [c] If patients have primary amenorrhea and normal secondary sexual characteristics, testing should begin with pelvic ultrasonography to check for congenital anatomic genital tract obstruction. |
| [d] Constitutional delay of growth and puberty is possible. |
| [e] Possible diagnoses include functional hypothalamic chronic anovulation and genetic disorders (eg, congenital gonadotropin-releasing hormone deficiency, Prader-Willi syndrome). |
| [f] Possible diagnoses include Cushing syndrome, exogenous androgens, congenital adrenal virilism, and polycystic ovary syndrome. |
| [g] Possible diagnoses include Turner syndrome and disorders characterized by Y chromosome material. |
| [h] Pubic hair may be sparse. |
| DHEAS = dehydroepiandrosterone sulfate; FSH = follicle-stimulating hormone; LH = luteinizing hormone. |
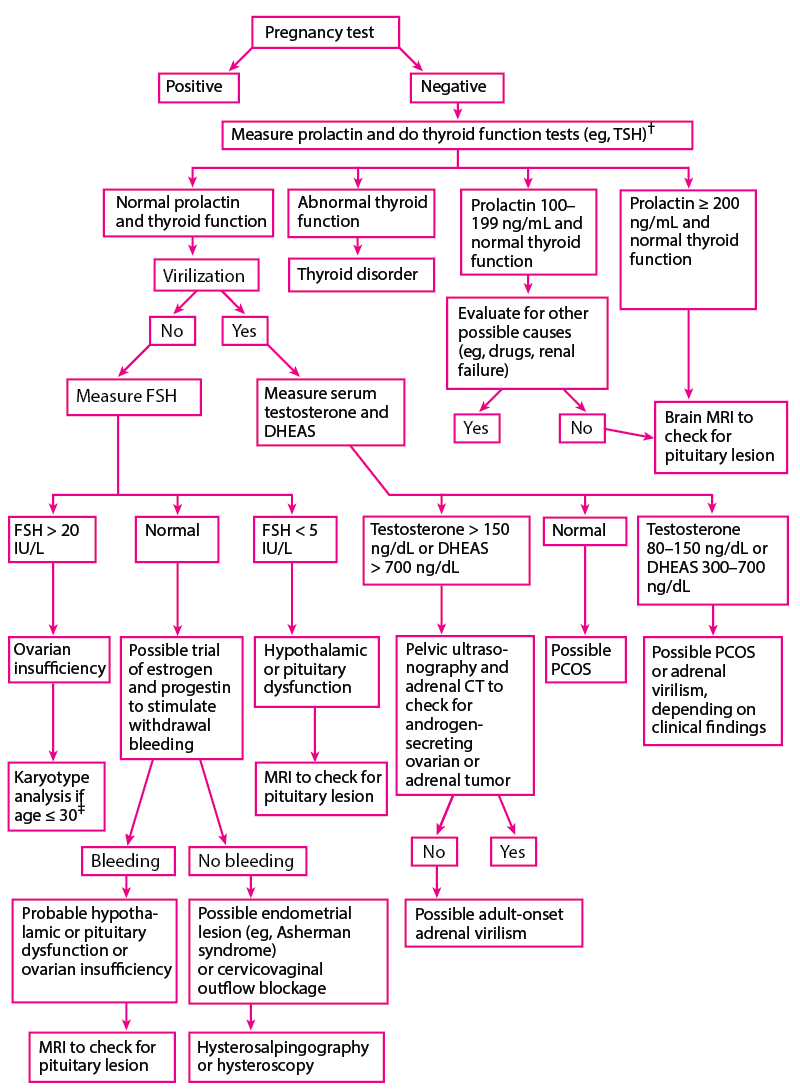 |
| * Normal values areDHEAS: 250–300 ng/dL (0.7–0.8 mcmol/L)FSH: 5‒20 IU/LKaryotype (female): 46,XXProlactin: 50 ng/mL (see below)Testosterone: 20–80 ng/dL (0.7–2.8 nmol/L)Although these values are representative, normal ranges may vary between laboratories.Prolactin 50–100 ng/mL is considered mildly elevated and is usually due to a drug adverse effect. Prolactin > 100 ng/mL is considered elevated and is more likely to be due to a tumor. |
| † Some clinicians simultaneously measure FSH and LH levels. |
| ‡ Clinicians should check for the presence of Y chromosome and Fragile X syndrome. |
| DHEAS = dehydroepiandrosterone sulfate; FSH = follicle-stimulating hormone; LH = luteinizing hormone; PCOS = polycystic ovary syndrome; TSH = thyroid-stimulating hormone. |
If symptoms or signs suggest a specific disorder, specific tests may be indicated regardless of what an algorithm recommends. For example, patients with abdominal striae, moon facies, a buffalo hump, truncal obesity, and thin extremities should be tested for Cushing syndrome. Patients with headaches and visual field defects or evidence of pituitary dysfunction require brain MRI.
If clinical evaluation suggests a chronic disease, liver and kidney function tests are done, and erythrocyte sedimentation rate (ESR) is determined.
Often, testing includes measurement of hormone levels; total serum testosterone or dehydroepiandrosterone sulfate (DHEAS) levels are measured only if signs of virilization are present. Certain hormone levels should be remeasured to confirm the results. For example, if serum prolactin is high, it should be remeasured; if serum follicle-stimulating hormone (FSH) is high, it should be remeasured monthly at least twice. Amenorrhea with high FSH levels (hypergonadotropic hypogonadism) suggests ovarian dysfunction; amenorrhea with low FSH levels (hypogonadotropic hypogonadism) suggests hypothalamic or pituitary dysfunction.
If patients have secondary amenorrhea without virilization and have normal prolactin and FSH levels and normal thyroid function, a trial of estrogen and a progestogen to try to stimulate withdrawal bleeding can be done (progesterone challenge test).
The progesterone challenge test begins by giving medroxyprogesterone 5 to 10 mg orally once a day or another progestogen for 7 to 10 days.
However, because this trial takes weeks and results can be inaccurate, diagnosis of some serious disorders may be delayed significantly; thus, brain MRI should be considered before or during the trial.
Mildly elevated levels of testosterone or DHEAS suggest polycystic ovary syndrome, but levels can be elevated in women with hypothalamic or pituitary dysfunction and are sometimes normal in hirsute women with polycystic ovary syndrome. The cause of elevated levels can sometimes be determined by measuring serum luteinizing hormone (LH). In polycystic ovary syndrome, circulating LH levels are often increased, increasing the ratio of LH to FSH.
Treatment is directed at the underlying disorder; with such treatment, menses sometimes resume. For example, most abnormalities obstructing the genital outflow tract are surgically repaired.
If a Y chromosome is present, bilateral oophorectomy is recommended because risk of ovarian germ cell cancer is increased.
Problems associated with amenorrhea may also require treatment, including